Shop By Category
-
(11 Products)
-
(5 Products)
-
(4 Products)
-
(1 Products)
-
(3 Products)
-
(4 Products)
-
(11 Products)
-
(7 Products)
Featured Products
Zirconia Milling Discs
-
-
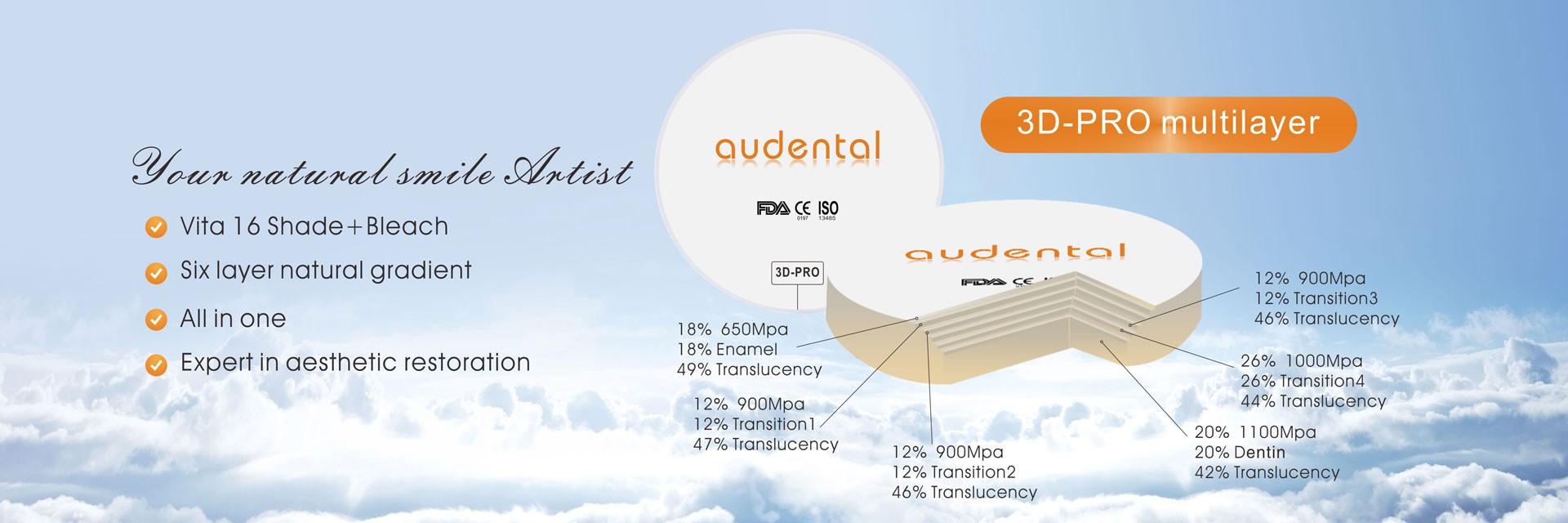
3D Pro Gradient Multilayer
$490HT White Dental Zirconia Disc
$184ST Multilayer Dental Zirconia Disc
$307-
ST Plus Multilayer Dental Zirconia Disc
$405ST Plus Preshaded Dental Zirconia Disc
$221ST Preshaded Dental Zirconia Disc
$221-
ST White Dental Zirconia Disc
$196UT Multilayer Dental Zirconia Disc
$429UT Preshaded Dental Zirconia Disc
$552-
PMMA Milling Discs
-
-
Multilayer PMMA Dental Milling Disc
$441Monochrome PMMA Dental Milling Discs
$172Pink PMMA Milling Disc
$294-
PEEK Milling Discs
-
-
Natural Color PEEK Milling Disc
$699Pink PEEK Milling Disc
$821Metal Milling Discs-
Cobalt-Chromium Alloy Disc – CAD/CAM Milling Disc for Frameworks & Bridges
$1,103Lithium Disilicate Blocks-
-
Lithium Disilicate Glass Ceramic Blocks – C14 & B40 (HT/LT) - Box of 5
$368Lithium Disilicate Press Ingots – HT / LT
$307Milling Machine Tools-
-
AuDental CAD/CAM Milling Burs - All Available Systems
$227Milling Burs – Roland Compatible (4mm / 6mm Shank)
$227Milling Burs – VHF Compatible (3mm Shank)
$196-
Milling Burs – Sirona Compatible (3mm Shank)
$196Milling Burs – Imes-icore Compatible (3mm / 6mm Shank)
$196Milling Burs – Arum Compatible (3mm / 6mm Shank)
$196-
Milling Burs – Amann Girrbach Compatible (3mm Shank)
$227Milling Burs – Wieland / Zenotec Compatible (3mm Shank)
$227Milling Burs – Zirkonzahn M5 Compatible (3mm / 5mm Shank)
$196-
Stain & Glaze
-
-
Zirconia Stain Coloring Liquid – Full Base Shade Kit – 16 Bottles
$1,324Zirconia Colouring Liquids – 16 VITA Shades
$112Stain / Glaze - Aesthetic Set
$4,512-
Free Ship Across AustraliaFree shipping on all ordersBulk Order Discount10% off on every order over $200024/7 Customer CareEmail Support
check_circle
check_circle
-
-
-
-
-
-
-
-
-